Find out information about single-replacement reaction. ZnCuCl2 Cu ZnCl2.

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
A chemical reaction in process Photo Credit.
/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
. A metal replaces another metal that is in solution. Chemistry single replacement reaction worksheet answer key 1. Cation replacement reaction A Cation Replacement Reaction.
Single Replacement Reactions A single replacement reaction occurs when one element replaces another in a single compound. A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound ie one element is replaced by the other in a compound. A single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products.
Cu h 2 o nr 10. Single-Replacement Reactions A single replacement reaction also known as a single displacement reaction occurs when one element in a molecule is swapped for another. Is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a.
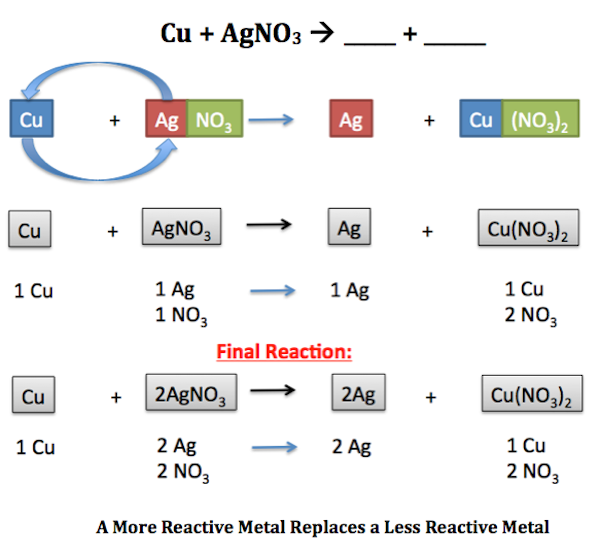
2al 3h 2 so 4 3 h 2 al 2 so 4 3 4. Zn 2agno 3 2ag znno 3 2 3. All metal displacement reactions are cation replacement reactions.
A cation is a positively charged ion or metal. There are two types of single replacement reactions. This type of a reaction can be depicted in the following manner.
Single replacement reaction displacement. A B-C B A-C. A single replacement reaction sometimes called a single displacement reaction is a reaction in which one element is substituted for another element in a compound.
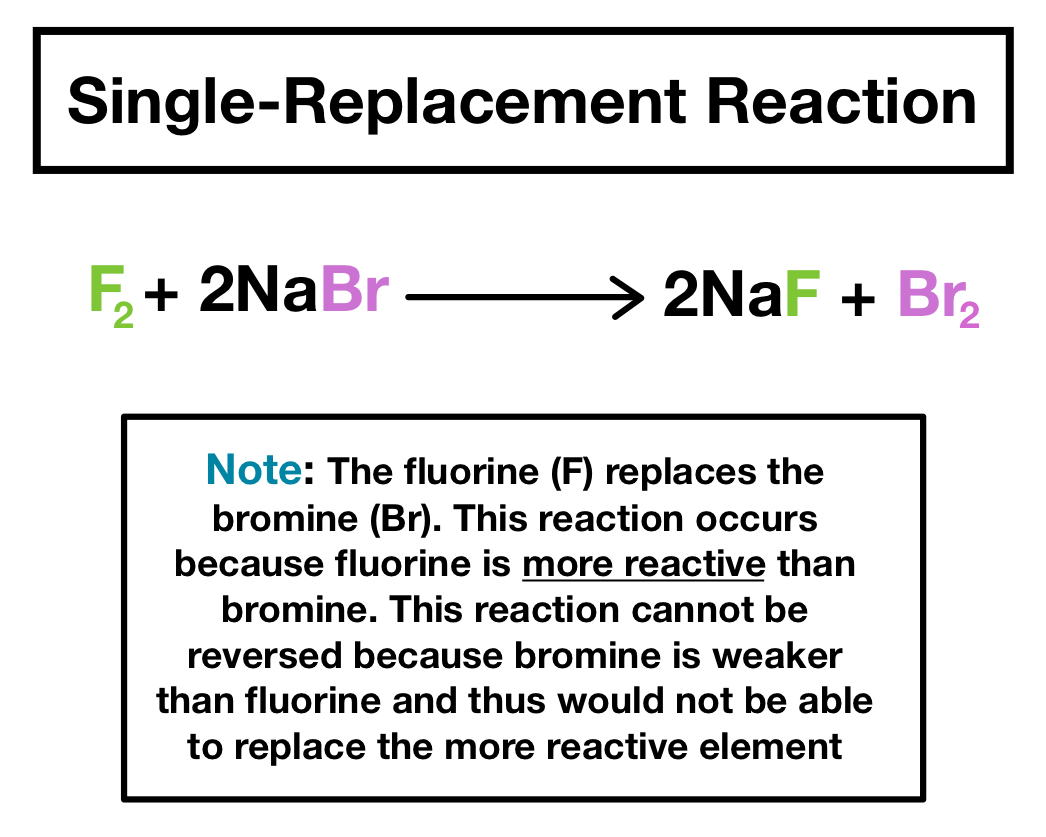
A BC B AC In this equation A represents a more reactive element and BC represents the original compound. 2al 3pbno 3 2 3pb 2alno. Cl 2 2 ki i 2 2kcl 5.
Cu ai 2 so 4 nr 11. Cu feso 4 nr 7. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions.
A single replacement reaction aka single displacement reaction will occur if M 1 cation is less reactive than M 2. The reactivity order corresponds to the reactivity series of the metals. 2li 2hoh h 2 2lioh 6.
Classification of single displacement reaction. Fe pbno 3 2 pb feno 3 2 9. Ag kno 3 nr 2.
Double-Replacement Reactions When components of two ionic compounds are swapped two new compounds are formed. A substitution or single displacement reaction is characterized by one element being displaced from a compound by another element. 2na 2hoh h 2 2naoh 8.
McGraw-Hill Dictionary of Scientific Technical Terms 6E Copyright 2003 by. Alright so were going to talk about single replacement reactions you might also. ShaiithShutterstock A replacement reaction is a type of chemical reaction in which one element replaces another in a compound.
ABC B AC. Neutralization precipitation and gas formation are types of double. Explanation of single-replacement reaction.
This can either be in the form of a single replacement reaction or a double replacement reaction. A chemical reaction in which an element replaces one element in a compound. Two examples are also sho.
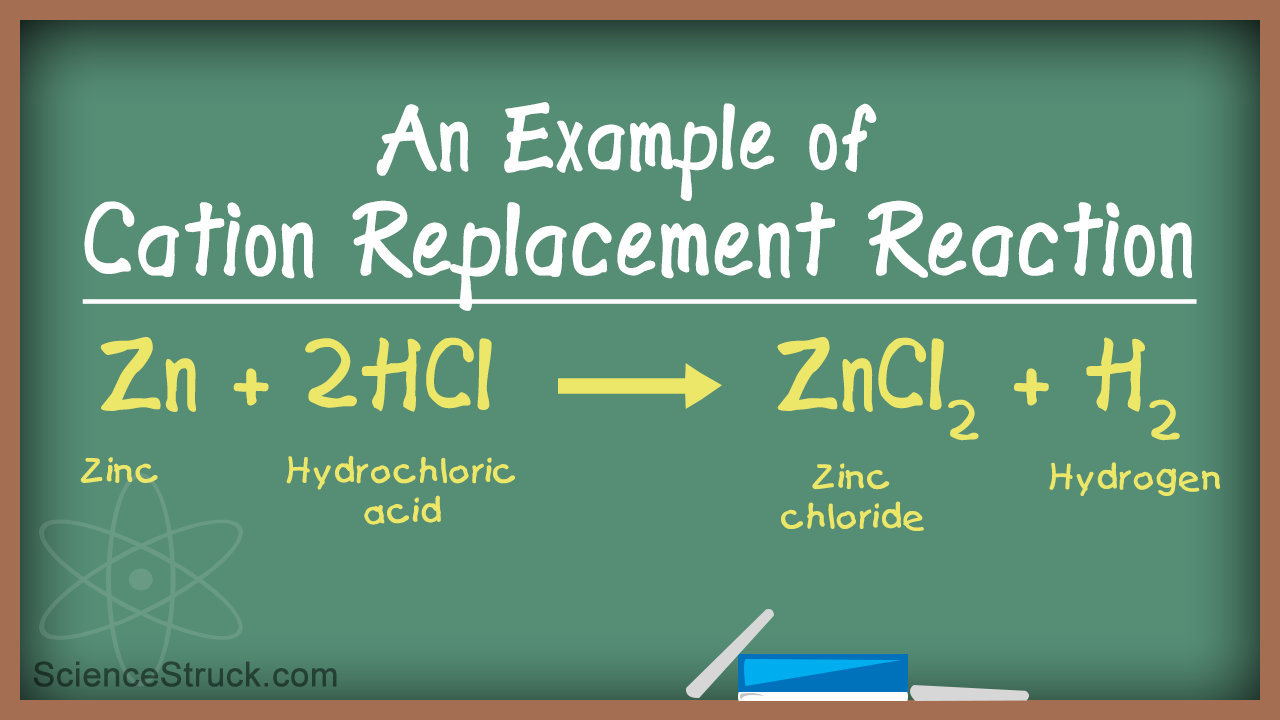
For example 2 HClaq Zns ZnCl 2 aq H 2 g. A single-replacement reaction A chemical reaction in which one element is substituted for another element in a compound. A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound.
In a single replacement reaction a single element replaces an atom in a compound producing a new compound and a pure element. The starting materials are always pure elements such as a pure zinc metal or hydrogen gas plus an aqueous compound. Describes the basics of single replacement reactions how to identify them predict the product and balance the chemical equation.
A single-displacement reaction also known as a single-replacement reaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that. A single displacement reaction is a specific type of oxidation-reduction reaction. This type of reaction has the general equation.
A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products. In a single replacement reaction one of the reactants is more reactive than the other which. Like double replacement reactions metals always replace metals and nonmetals always replace nonmetals in a compound.
In this reaction one cation replaces another one from its solution. 2 K 2 H 2 O 2 K O H H. Single replacement reactions or single displacement reactions involve the replacement of an atom or an ion from one compound by a more reactive compound.
Looking for single-replacement reaction.
/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
Types Of Chemical Reactions With Examples

Double Replacement Reaction Definition And Examples
Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
Single Replacement Reactions Definition Examples Expii

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube

Single Replacement Reaction Definition And Examples

0 comments
Post a Comment